Well over a year ago, I wrote about a homemade herbicide containing salt, vinegar, and dish soap.
“Many of you have probably seen it posted to Facebook or Twitter or Pinterest, or on your favorite home gardening site. One of my favorite descriptions calls it a “magical, natural, weed killing potion.”
That particular potion certainly kills weeds, but it isn’t natural (and it certainly isn’t chemical-free). It contains dish soap and vinegar, both of which are synthesized industrially, so it isn’t natural by most definitions of the word. That’s disappointing, because people really yearn for a natural weedkiller. They want to kill the weeds around their homes and in their gardens, but they don’t like the idea of using a synthetic pesticide. Most people (including me) would prefer to use something natural, all else being equal. Unfortunately, there are very few truly natural products that work as effective herbicides.
That being said, I’d like to introduce you to a fascinating chemical named bilanaphos. In the early 1970’s, bilanaphos was discovered independently by two different laboratories, one in Germany and the other in Japan. Both groups isolated this chemical from Streptomyces bacteria; S. viridochromogenes in Germany, and S. hygroscopicus by the Japanese group. Bilanaphos is produced naturally by these naturally occurring bacteria. So, by nearly any definition, bilanaphos is natural.
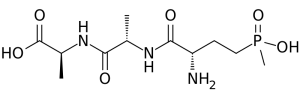
The scientists in Germany and Japan both learned early-on that bilanaphos had strong weed-killing properties; when it was applied to plants, the plants died. Upon further investigation, scientists in the German group recognized that only part of the full bilanaphos chemical was required for herbicidal activity. In fact, when bilanaphos enters the plant, about half of the molecule is quickly chopped off, leaving behind a smaller molecule – phosphinothricin. It is this smaller molecule that acts as a herbicide in the plant.
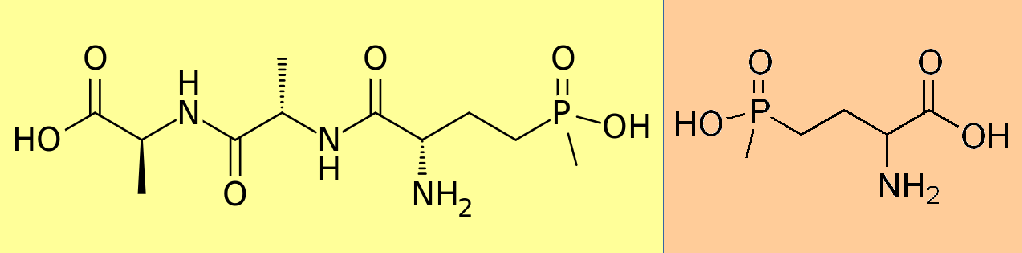
So we have a natural compound (bilanaphos) that is converted naturally by plants to another compound (phosphinothricin) that works very effectively as a herbicide. And it turns out that some Streptomyces species naturally produce a small amount of phosphinothricin also. That sounds very much like a natural herbicide, right? Not so fast…
Phospinothricin (better known in the US as glufosinate) is widely used as a herbicide today. It is the active ingredient in herbicides like Rely (mostly used in tree and vine crops), and Liberty (most commonly used in conjunction with Liberty Link crops). But even though the chemical occurs naturally, and was first discovered by extracting it from naturally occuring bacteria, the commercial herbicide is produced synthetically. So it is not considered a ‘natural’ herbicide.
The story of phosphinothricin, while very interesting, is not unique. A huge number of scientists around the world are searching nature to find new chemicals that have antibiotic, pesticidal, or other useful properties. Between 1997 and 2010, USDA scientists estimate that about 69% of all new pesticide active ingredients registered by the EPA were either natural products, synthetic products derived from natural sources (like phospinothricin), or biological in nature. For example, another commonly used corn herbicide was discovered after an initial observation that few plants could grow underneath a red bottlebrush bush in a garden. But weed killers are actually the smallest component (less than 7%) of these new pesticides of natural origin; around 30% of new insecticide or fungicide active ingredients are either natural products or natural product-derived.
Currently, the FDA is struggling to define the word natural on food labels. It is an often-used marketing term with no clear definition. It may be even more difficult to define when discussing pesticides. As the phosphinothricin example shows, the lines between natural and synthetic can get blurred quickly. Is it natural because it occurs in nature? Or does it have to be physically extracted from nature to be considered natural?
The ‘natural or not‘ distinction can distract from what is really important when discussing pesticides. If the compound is structurally the same, the naturally occurring and the synthetically produced versions will share the same properties. The properties of the compound are far more important, in my opinion, than the source of the compound. Is the pesticide safe for applicators and the environment? Does it break down quickly in the environment to non-toxic products? If so, then I’m much less worried about whether it is natural or not, regardless of how we define natural.
But there are questions related to the source of the product that can be important. In particular, which has a greater impact, synthesis in the lab? or extraction from natural sources? I rarely hear discussions related to this question, but this is among the most important questions related to natural products (provided they are deemed safe). If we can efficiently extract a renewable resource from nature, and avoid the energy and fossil fuel requirements of synthetic production, then a naturally produced product sounds pretty good to me. But if extracting something from nature means we’ll have a greater negative impact on the environment than we would producing it in a factory, then please give me the synthetic version.
References:
Hoerlein (1994) Glufosinate (Phosphinothricin), A Natural Amino Acid with Unexpected Herbicidal Properties. p 73-145 in Reviews of Environmental Contamination and Toxicology (Vol 138)
Dayan et al. (2011) Rationale for a natural products approach to herbicide discovery. Pest Management Science. 68:519–528
Cantrell et al. (2012) Natural Products as Sources for New Pesticides. Journal of Natural Products. 75:1231-1242.


Comments are closed.